ZimFact is publishing a series of fact guides on Covid-19 vaccines as a contribution to the provision of factual information around a pandemic surrounded by so much misinformation.
The Sinopharm vaccine
China’s Sinopharm vaccine received World Health Organisation (WHO) approval for emergency use on Friday, 7 May 2021. Sinopharm joins vaccines produced by Pfizer-BioNTech, AstraZeneca, Moderna and Johnson & Johnson on the list of WHO-approved COVID-19 vaccines.
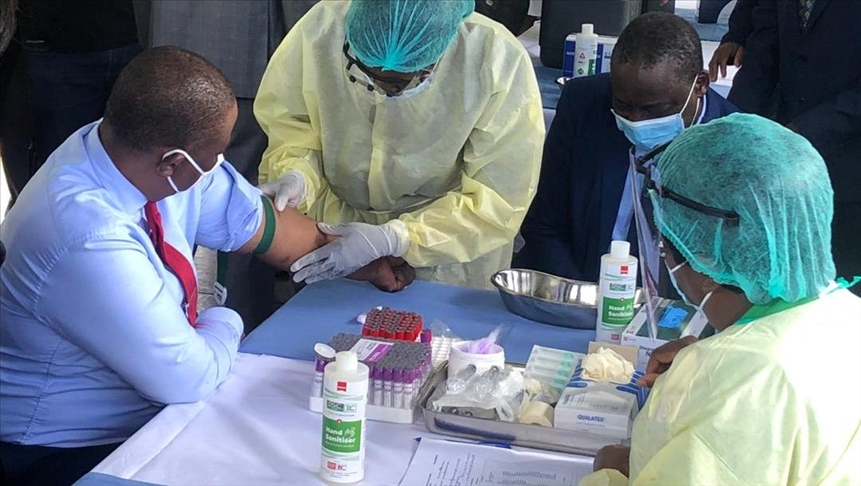
Zimbabwe approved and began using Sinopharm back in February, when it took delivery of the first of two donated 200,000 dose batches. The Zimbabwe government says it plans to buy more Sinopharm doses from China as it bids to vaccinate 10 million people.
The Sinopharm vaccine was the first to be used in Zimbabwe, which has also licenced another Chinese vaccine, CoronaVac (Sinovac), India’s Covaxin and Russia’s Sputnik V for use.
Like CoronaVac, the Sinopharm vaccine was developed using the traditional method, by means of an inactivated virus. In an inactivated vaccine, the virus – in this case the coronavirus causing COVID-19 – is killed or modified in such a way that it is unable to replicate. It cannot cause disease and is, therefore, suitable for those with a compromised immune system.
The inactivation is usually done using heat, radiation or chemicals to destroy the pathogen’s genetic material, which stops it from replicating.
While inactivated vaccines can trigger a strong immune response,
They usually require a person to have booster shots to ensure ongoing protection.
Who developed it?
The state-owned China National Pharmaceutical Group (Sinopharm) and the Beijing Institute of Biological Products (BIBP) jointly developed the Sinopharm vaccine under Sinopharm’s vaccine and bioscience subsidiary, China National Biotec Group (CNBG).
What is its formal name?
Although widely known simply as the Sinopharm vaccine, the company which developed the vaccine calls it the “Sinopharm CNBG COVID-19 vaccine”.
The WHO Strategic Advisory Group of Experts (SAGE), which advises the global body on immunisation, calls it the “COVID-19 vaccine BIBP.”
How was it developed?
From three coronavirus variants obtained from patients in Chinese hospitals, researchers picked one that showed ability to quickly multiply in monkey kidney cells grown in bioreactor tanks. Once large stocks of the coronaviruses were developed, they were inactivated using a chemical called beta-propiolactone. While the inactivated coronaviruses could no longer replicate, their proteins, including the spike, remained intact.
The researchers then drew off the inactivated viruses and mixed them with a tiny amount of an aluminum-based compound called an adjuvant. Adjuvants stimulate the immune system to boost its response to a vaccine.
How does it fight SARS-CoV-2, the virus which causes COVID-19?
As an inactivated virus vaccine, CoronaVac works by using killed viral particles to expose the body’s immune system to the virus, but without risking a serious disease response.
The body responds by generating antibodies, helping the immune system to fight infection by a live coronavirus.
Storage
Like most conventional vaccines, the Sinopharm vaccine is stored within the 2-8 degrees celsius temperature range.
Efficacy and effectiveness
Vaccine efficacy and effectiveness are often confused and used interchangeably.
Efficacy measures the performance of a vaccine measured during a clinical trial, while effectiveness refers to how well the vaccine does in the real world.
WHO experts who reviewed the Sinopharm vaccine’s data say its efficacy for symptomatic and hospitalized disease was estimated to be 79%, all age groups combined.
The experts noted that while few persons aged above 60 years took part in the Sinopharm clinical trials, resulting in limited efficacy data for that age-group, “there is no theoretical reason to believe that the vaccine has a different safety profile in older and younger populations.”
Sources: World Health Organisation, Sinopharm Group, New York Times, Washington Post
Do you want to use our content? Click Here