ZimFact is publishing a series of fact guides on COVID-19 vaccines as a contribution to the provision of factual information around a pandemic surrounded by so much misinformation.
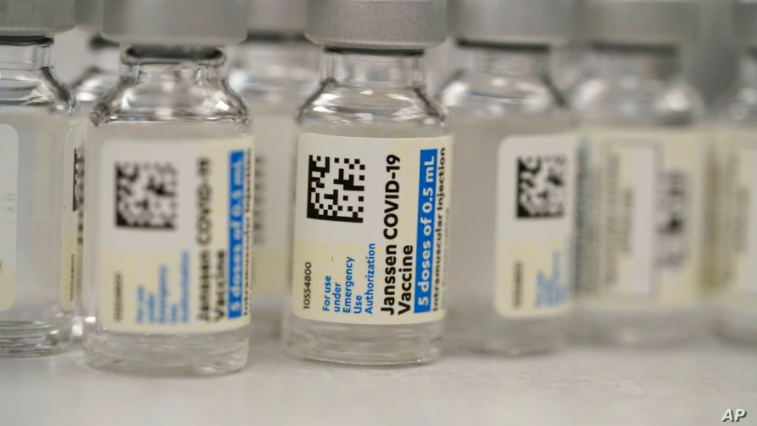
The Johnson & Johnson vaccine
The World Health Organisation authorised the emergency use of the Johnson & Johnson single-dose vaccine in March 2021. However, the rollout of the vaccine has been dogged by safety concerns associated with blood clots.
In April, the United States’ Centres for Disease Control and the Food and Drug called for a pause in the use of the Johnson & Johnson vaccine after six recipients of the vaccine developed a rare disorder involving blood clots within six to 13 days after being inoculated.
Ten days later, the two agencies recommended the resumption of Johnson & Johnson vaccinations after determining “that the available data show that the vaccine’s known and potential benefits outweigh its known and potential risks in individuals 18 years of age and older.”
In May, the COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS) said:
“Current evidence suggests a plausible causal association between the J&J COVID-19 vaccine and TTS. Clinically, the features of TTS following vaccination with this vaccine appear similar to those observed following another adenoviral vectored vaccine, the AstraZeneca COVID-19 vaccine.”
TTS is thrombosis with thrombocytopenia syndrome, referring to blood clots (thromboembolic events) and low platelets (thrombocytopenia).
Similar events have been associated with the AstraZeneca COVID-19 vaccine, according to the WHO.
However, the WHO experts concluded that “the benefits of the J&J COVID-19 vaccine continue to outweigh the risks of TTS. As the only single dose COVID-19 vaccine approved for use to date, the vaccine may be an important tool for accessing difficult-to-reach populations, thus playing a key role in preventing infections and reducing deaths across the world.”
Zimbabwe, which has so far only approved four vaccines, two from China and one each from Russia and India, is yet to register the Johnson & Johnson vaccine, which the country was scheduled to receive in August under the African Union’s COVID-19 vaccination programme.
“The government of Zimbabwe is not yet ready to participate in the August (2021) allocation as measures are still being put in place to establish the cold chain management framework for the vaccines, as well as on management of the anticipated adverse effects of the vaccines following inoculation,” Treasury permanent secretary George Guvamatanga wrote to a continental bank funding the procurement plan on June 2.
Who developed it?
Janssen Pharmaceutica, a Belgium-based division of Johnson & Johnson, developed the vaccine in collaboration with Beth Israel Deaconess Medical Center. Johnson & Johnson is an American pharmaceutical company founded in 1886. Johnson& Johnson acquired Janssen in 1961.
What is its formal name?
The vaccine has a rather unwieldy official name – Ad26.COV2-S. But it is widely known as either the Janssen vaccine or the Johnson & Johnson (J&J) vaccine.
How was it developed?
The Johnson & Johnson vaccine is an adenovirus vaccine. This means it uses an adenovirus, a virus which causes common flu, as a delivery vehicle.
The coronavirus causing COVID-19 has a surface studded with protein spikes which are targeted by many vaccines and treatments.
The Johnson & Johnson vaccine is based on the virus’s genetic instructions for building the spike protein. But unlike the Pfizer-BioNTech and Moderna vaccines, which store the instructions in single-stranded RNA, the Johnson & Johnson vaccine uses double-stranded DNA.
The researchers added the gene for the coronavirus spike protein to another virus called Adenovirus 26. Adenoviruses are common viruses that typically cause colds or flu-like symptoms. The Johnson & Johnson team used a modified adenovirus that can enter cells but can’t replicate inside them or cause illness.
Adenovirus vaccines such as Johnson & Johnson, AstraZeneca and Sputnik V are the result of decades of research into the technology. An adenovirus-based vaccine for ebola, also developed by Johnson & Johnson, was approved for general use in July 2020. Trials are also being run for other adenovirus-based vaccines for HIV and Zika.
How does it fight SARS-Cov-2, the virus which causes COVID-19?
After the vaccine is injected into a person’s arm, the adenovirus in the Johnson & Johnson vaccine delivers DNA to the body’s cells, with instructions to make the spike protein. After the body’s cells produce the spike protein, the immune system creates antibodies toward the spike protein, providing protection from infection.
Storage
Adenovirus-based vaccines for Covid-19 more durable than mRNA vaccines from Pfizer and Moderna. The adenovirus’s tough protein coat helps protect the genetic material inside. As a result, the Johnson & Johnson vaccine can be refrigerated for up to three months at 2–8° Celsius.
Efficacy and effectiveness
Vaccine efficacy and effectiveness are often confused and used interchangeably.
Efficacy measures the performance of a vaccine measured during a clinical trial, while effectiveness refers to how well the vaccine does in the real world.
According to the WHO, clinical trials found that the Johnson & Johnson COVID-19 vaccine had an efficacy of 66.9% against symptomatic moderate and severe SARS-CoV-2 infection.
The vaccine was also found to have an efficacy of 85.4% against severe disease and hospitalization, 28 days after inoculation.
Sources: WHO, Johnson & Johnson/Janssen Pharmaceutica, University of Nebraska Medical Centre, New York Times, Washington Post, Reuters
Do you want to use our content? Click Here