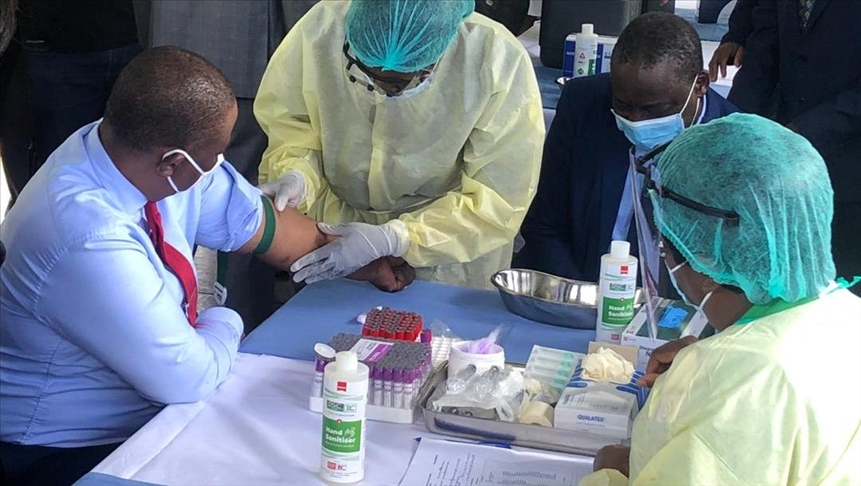
CLAIM: The United States Food and Drug Administration (FDA) guidelines advise a two week waiting period before people who get vaccines such as AstraZeneca, Johnson & Johnson and Sinopharm can donate blood.
Source: Health Times, a Zimbabwean online health news service.
VERDICT: INCORRECT. The FDA’s guidance says individuals who received a nonreplicating, inactivated, or mRNA-based COVID-19 vaccine can donate blood without a waiting period. Only recipients of live-attenuated viral COVID-19 vaccines require a two week waiting period before they can donate blood, according to the guidance.
Citing FDA guidelines in an article on Zimbabwe’s diminished blood stocks, the Health Times reported that people inoculated against COVID-19 using AstraZeneca, Johnson & Johnson and Sinopharm vaccines could only donate blood two weeks after taking shots.
“According to the Food and Drug and Drug Administration (FDA), individuals who get jabs from vaccines such as the AstraZeneca, Johnson & Johnson and the Sinopharm which are replication defective virus vaccines must wait two weeks before donating blood,” Health Times reported.
“However, if someone got an inactivated or RNA based COVID-19 vaccine, like the ones manufactured by Moderna and Pfizer, there is no waiting period to donate blood. The Red Cross recommends that if a person does not know which type of COVID-19 vaccine they received they must wait four weeks before donating.”
FDA guidance
Guidance issued by the FDA on 19 January 2021 says “individuals who received a nonreplicating, inactivated, or mRNA-based COVID-19 vaccine can donate blood without a waiting period.”
It adds that “individuals who received a live-attenuated viral COVID-19 vaccine, refrain from donating blood for a short waiting period (e.g., 14 days) after receipt of the vaccine.”
The vaccines cited by Health Times – Sinopharm, AstraZeneca and Johnson & Johnson – all fall under the FDA’s category of non-replicating or inactivated vaccines.
Sinopharm is an inactivated vaccine made from a virus killed off by radiation or chemicals, which has no capacity to replicate but has the ability to trigger an immune response.
Both AstraZeneca and Johnson & Johnson are non-replicating viral vaccines based on a weakened common cold virus that readily infects human cells but is incapable of causing disease.
The American Red Cross, which says it is following FDA blood donation eligibility guidelines for those who receive COVID-19 vaccines, says there is no waiting time for most individuals who received a COVID-19 vaccine as long as they are symptom free and feeling well at the time of donation.
“There is no deferral time for eligible blood donors who are vaccinated with an inactivated or RNA based COVID-19 vaccine manufactured by AstraZeneca, Janssen/J&J, Moderna, Novavax, or Pfizer,” the American Red Cross says.
“Eligible blood donors who received a live attenuated COVID-19 vaccine or do not know what type of COVID-19 vaccine they received must wait two weeks before giving blood.”
CONCLUSION:
The assertion that FDA guidelines recommend a waiting period of two weeks before people inoculated using AstraZeneca, Johnson & Johnson and Sinopharm vaccines can donate blood is untrue. FDA advice says only recipients of live attenuated vaccines, of which none is currently being administered, should wait for about two weeks before donating blood.
Do you want to use our content? Click Here