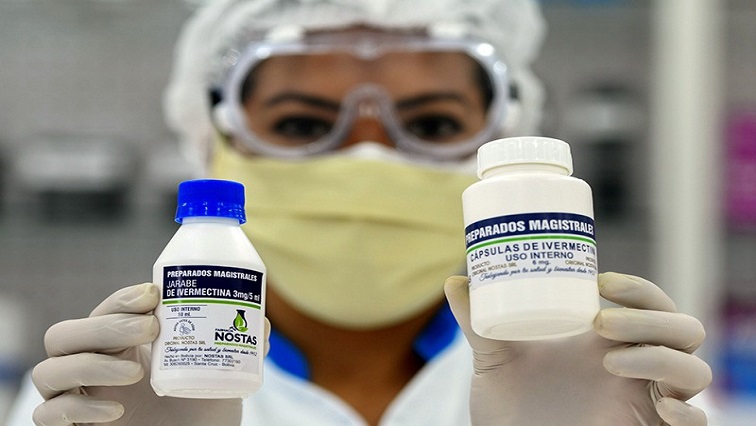
On June 28, 2021, the Medicines Control Authority of Zimbabwe (MCAZ), which regulates the approval and registration of medical drugs in the country, announced it had approved the use of ivermectin to treat COVID-19, as part of what the regulator called “operational research.”
What is ivermectin?
Ivermectin is an anti-parasitic drug developed by Japan’s Kitasato Institute and pharmaceutical multinational Merck & Co in the 1970s. Ivermectin is a broad spectrum drug, meaning it is effective against a wide range of diseases.
What does it treat?
Ivermectin is used in animal health against a range of internal and external parasites. It is also approved by the World Health Organisation for the treatment of river blindness and scabies, among other ailments.
Ivermectin and COVID-19
The WHO’s guidance, issued on March 31, 2021, states that:
“The current evidence on the use of ivermectin to treat COVID-19 patients is inconclusive.”
Until more data is available, the WHO recommends that the drug only be used within clinical trials.
In many jurisdictions, including the United States, authorities are against the use of ivermectin for COVID-19.
The US Food and Drug Administration warns against the use of ivermectin against COVID-19.
“While there are approved uses for ivermectin in people and animals, it is not approved for the prevention or treatment of COVID-19,” the FDA says.
“Any use of ivermectin for the prevention or treatment of COVID-19 should be avoided as its benefits and safety for these purposes have not been established.”
However, an April 2020 research article by Australian scientists said laboratory tests had shown that ivermectin “is an inhibitor of the COVID-19 causative virus.”
The scientists, however, urged further investigations into ivermectin.
Divided expert opinion
In Zimbabwe, as in many other countries, ivermectin has divided expert opinion.
On January 8, 2021, the MCAZ issued a statement warning against the use of veterinary ivermectin.
On 20 January, 2021, the Medical and Dental Practitioners Council of Zimbabwe (MDCZ), a statutory body regulating the practice of medicine and dentistry in the country, issued a statement warning against the use of ivermectin.
“There has been widespread advertising on social media by some medical practitioners claiming to be able to treat COVID-19 using ivermectin, doxycycline and nanosilver,” “We would like to make the record straight that ivermectin is an old drug used to treat parasitic infections and is not currently registered by the Medicines Control Authority of Zimbabwe for the treatment of COVID-19 patients,” the council said.
“There are several ongoing studies looking at the safety and effectiveness of this drug in COVID-19 treatment. So far, no evidence has come out to warrant its registration and widespread use to treat COVID-19 patients.”
But a 24 January, 2021 letter by the College of Primary Care Physicians asked the government to allow the use of ivermectin, and nanosilver, for treatment of COVID-19 patients.
“Due to our constant perusal of the medical evidence we noticed the signals around ivermectin in August 2020, largely in third world countries like ours. We feel comfortable using this drug which has been around for 40 years, is on the WHO essential drugs list and has an excellent safety profile,” the 23 physicians wrote.
“We understand that it is currently not registered in Zimbabwe but we will fill out Section 75 forms for non-registered drugs all the time in the course of our practice. We heard from colleagues in South Africa who were using ivermectin with excellent results, although they too were losing occasional patients who presented late.”
Zimbabwe’s latest position on ivermectin
The MCAZ says it has not allowed wide-spread use of ivermectin for COVID-19 treatment. Rather, the regulator is giving a qualified nod to health professionals, within a strict framework, to prescribe, procure and dispense the drug.
“The Medicines Control Authority of Zimbabwe is aware of media reports suggesting that the Authority had issued a blanket approval for the widespread use of ivermectin human formulations for the prevention and treatment of COVID-19. Several critical studies are still being undertaken to evaluate ivermectin’s safety and efficacy in treating the COVID-19 infection in vivo (in human bodies, as opposed to in vitro, in labs), but there has been no conclusive evidence yet to support its use,” MCAZ said in its 28 June, 2021 statement.
“In an effort to add more options to the list of medicines with potential evidence-based therapeutic value in the prevention and or treatment of COVID-19, the Authority sought approval from the Secretary for Health and Child Care to establish a framework that would provide guidance on the use of ivermectin in COVID-19, in the form of operational research.”
Under the framework, only authorised pharmaceutical wholesalers will be allowed to import and supply ivermectin to authorised health facilities, medical practitioners and pharmacies.
“Members of the public are strongly urged to desist from self-prescribing and sourcing unapproved medication from unapproved sources,” the MCAZ added.
The authority will also gather information on whether patients are realising clinical benefits from the use of ivermectin, while also monitoring for any side effects.
Do you want to use our content? Click Here